
The molecular geometry of XeF2 is linear, meaning that the fluorine atoms are attached to the xenon atom in a straight line. Explanation of the linear-shaped geometry of XeF2 It is an interesting molecule to study because its nature, whether polar or nonpolar, can be determined by analyzing its molecular geometry and the polarity of its bonds. XeF2, or xenon difluoride, is a chemical compound composed of xenon (Xe) and fluorine (F) atoms. The electronegativity difference between xenon and fluorine is negligible, contributing to the nonpolar nature of XeF2.The molecule has a linear geometry and symmetrical electron distribution, resulting in a cancellation of dipole moments.
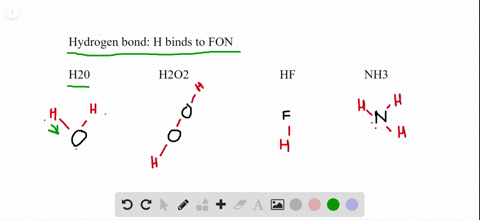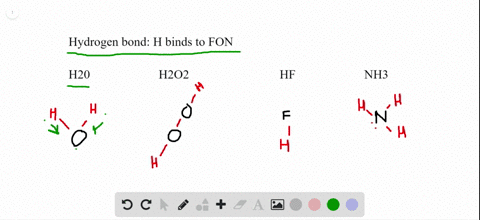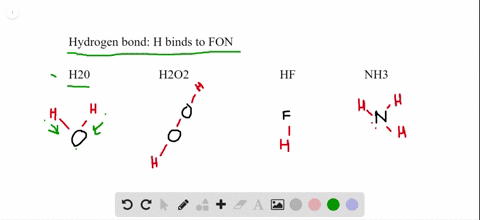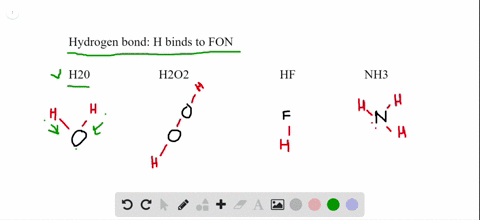
So, let’s delve into the world of XeF2 and unravel its polar or nonpolar mystery. By understanding the molecular structure and properties of XeF2, we can gain insights into its behavior and applications in various fields. In this article, we will explore the polar or nonpolar nature of XeF2 and examine the factors that determine its polarity. Polarity refers to the distribution of electrons in a molecule and whether it has a positive and negative end. It is an interesting molecule to study because its polarity is a subject of debate among scientists. XeF2 is a chemical compound composed of xenon and fluorine.


 0 kommentar(er)
0 kommentar(er)
